London, United Kingdom – Intract Pharma (‘‘Intract’’) today announced the appointment of Srini Ramanathan, Ph.D. as a product development advisor. Dr Ramanathan brings a wealth of drug development expertise across a wide range of diseases and will provide strategic development expertise to Intract’s team as the company moves forward towards taking novel orally delivered antibody therapeutics in immunology towards the clinic.
Dr Ramanathan is Chief Development Officer at Roivant overseeing R&D for diligence around business opportunities, new company creation and stand-up. He was previously at Horizon Therapeutics where he served as Senior Vice President of Research and Development Sciences and Site Head of the South San Francisco office. At Horizon, Dr. Ramanathan was responsible for various scientific functions across discovery and development supporting pipeline expansion, regulatory submissions, and business development activities. Earlier in his career, he served in leadership roles at AbbVie and Gilead Sciences. He has made significant contributions to the global approval of several medicines in virology, oncology, and autoimmune disease, and has presented/published his research extensively.
‘’We are delighted to have Srini join us as an advisor. With his vast experience in R&D and drug development, Srini will be a great addition as our team works towards taking our lead oral anti-TNFα mAb towards the clinic together with Tharimmune, as well as expand our pipeline of next generation of oral biologics’’
Vipul Yadav, CEO Intract Pharma.
‘’I am excited to advise the Intract team in developing new treatments with their innovative and unique oral antibody/biologics delivery technology’’
Dr Ramanathan
Intract Pharma is a biopharmaceutical company developing disruptive oral antibody delivery solutions to significantly improve the efficacy and safety of emerging and established protein therapeutics, as well as improve patient experience and outcomes in inflammation and immunology indications. Its platform leverages the advantage of precision targeting of large proteins and antibodies to the colon, while also protecting the biologics from enzymatic breakdown, allowing tissue/systemic uptake to create next-generation oral antibody medicines. For more information visit: www.intractpharma.com
Roivant’s mission is to improve the delivery of healthcare to patients by treating every inefficiency as an opportunity. Roivant develops transformative medicines faster by building technologies and developing talent in creative ways, leveraging the Roivant platform to launch ‘Vants’ – nimble and focused biopharmaceutical and health technology companies. For more information, please visit www.roivant.com.
Dr Vipul Yadav
CEO
LONDON and BRIDGEWATER, N.J. / ACCESSWIRE / March 25, 2025 – Intract Pharma and Tharimmune, Inc. (Nasdaq: THAR), a clinical-stage biotechnology company focused on immunology and inflammation, today announced positive preclinical results for its novel oral antibody, TH023. In a murine model, a proprietary protease enzyme stabilization platform demonstrated successful delivery of infliximab, a tumor necrosis factor-alpha (TNF-α) inhibitor, in serum with concentrations detected being significantly higher than the standard serum trough concentration needed for antibody efficacy in immunology indications via injection (~3-5µg/ml)1. These findings represent a significant step towards developing a more convenient and potentially patient-preferred alternative to currently available infliximab treatments, which are administered via intravenous infusion or subcutaneous injection.
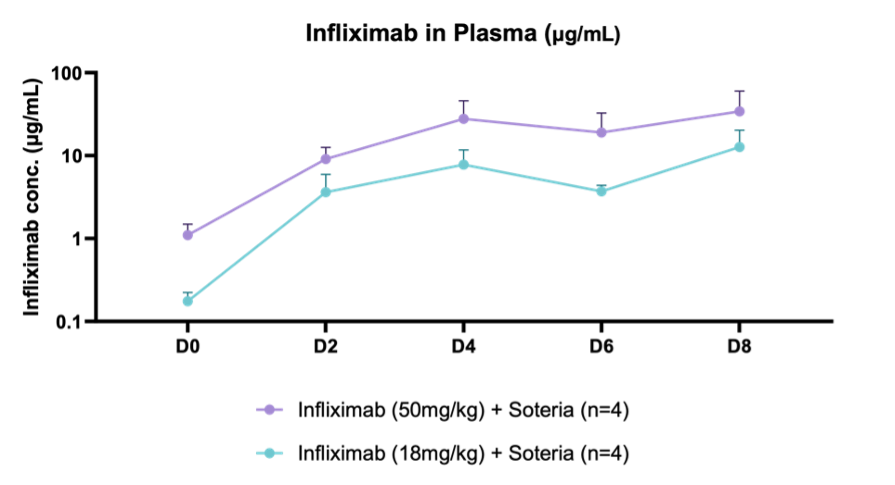
Key findings of the preclinical evaluation includes demonstrating enzymatic protection of infliximab against human colon enzymes ex vivo using fresh fecal samples from healthy subjects utilizing the SoteriaTM platform, a proprietary formulation of natural amino acids (data not shown).
Furthermore, successful delivery of TH023 in-vivo into both local colonic tissue and systemic circulation was shown following intra-duodenal QD dosing for 1 week in a healthy mouse model at two doses of infliximab. This data shows the potential of the delivery platform to allow for both local delivery of the antibody precisely in the large intestine tissue through enzymatic stabilization, as well as systemic circulation, which is an ideal PK profile for targeting local gastrointestinal (GI) diseases such as IBD as well as systemic inflammatory diseases.
The mechanism by which the antibody transcytosis occurs in the GI tract was shown to be a combination of passive and neonatal FcRn mediated active transport, a receptor that’s highly expressed in the distal intestinal epithelial cells.
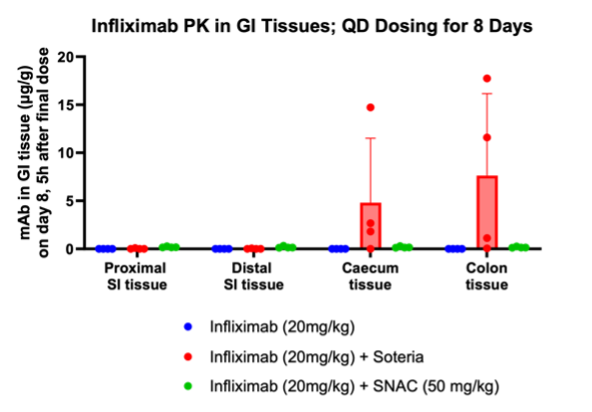
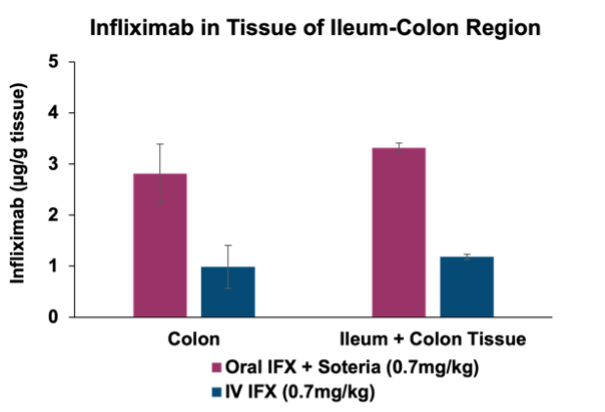
Furthermore, the study demonstrated that tissue penetration of infliximab in combination with the enzyme stabilization platform was superior to a traditional permeation enhancer sodium N-(8-[2-hydroxylbenzoyl] amino) caprylate (SNAC) which has been used to enhance the absorption of GLP-1 peptides such as semaglutide. Utilization of SNAC to protect infliximab from enzymatic degradation or permeation enhancement did not result in tissue or serum concentrations suggesting standard off-the-shelf oral peptide delivery technologies do not work for oral delivery of monoclonal antibodies. We tested two other standard permeation enhancer technologies (sodium caprate and labrasol) which gave the same result (data not shown).
The Company announced last year through a partnership with Intract Pharma an exclusive license to INT-023 (now TH023), an oral anti-tumor necrosis factor alpha monoclonal antibody, infliximab. Tharimmune licensed global development and commercialization rights (outside of South Korea) to Intract Pharma’s Soteria® and Phloral® delivery platform along with an existing supply agreement for infliximab to be used in the oral product development program. Traditionally administered through intravenous infusions, oral delivery of antibodies is challenging due to the complexity of navigating such large molecules through the gastrointestinal tract. An oral route of administration holds potential to improve patient compliance and quality of life, while also reducing the burden on the healthcare system associated with long-term intravenous therapy.
“We are extremely encouraged by these results, which validate the potential of our partnership with Intract and the platform to deliver complex biologic molecules like infliximab orally,” said Randy Milby, CEO of Tharimmune. This represents a potential major milestone in our mission to develop more patient-friendly and accessible treatment options for chronic inflammatory diseases, addressing a multi-billion dollar market. While these are early findings, they provide a strong foundation for further development and eventual clinical trials.”
“These positive preclinical results are an important milestone as we work towards taking this highly validated anti-TNFα antibody in a first-in-class oral pill form using our delivery platform in severe autoimmune indications where patients currently only have injectable options”
Vipul Yadav, CEO of Intract
Through the Company’s existing partnership with Intract the data announced today enables for the targeted delivery of antibody therapeutics directly to the colon or small intestine. By leveraging Intract’s platform, Tharimmune aims to enhance the effectiveness of TNF-α inhibitors such as infliximab through precision delivery that maximizes proteolytic stabilization and tissue permeation. This novel approach offers significant potential for directly addressing inflammatory conditions within the gastrointestinal tract, including inflammatory bowel disease, as well as systemic inflammatory disorders where TNF-α plays a critical role in disease progression. Tharimmune plans to optimize the formulation and dosing regimen and prepare to conduct a first-in-human clinical trial with TH023 in the next 12 months.
Infliximab, a TNF-alpha inhibitor, is a widely used biologic for the treatment of several chronic inflammatory diseases, including Crohn’s disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. Globally, infliximab (including biosimilars) generated approximately $6.3 billion in sales in 2022, demonstrating the significant market for this therapy. However, current administration routes require frequent visits to healthcare facilities, which can be burdensome for patients and contribute to significant healthcare costs. Experts suggest the market for infliximab could rise to 9 billion USD within 10 years. An oral formulation of infliximab has the potential to improve patient convenience and compliance and eliminating the need for injections or infusions which could significantly improve the patient experience and potentially lead to better treatment adherence. Oral administration could reduce the need for clinic visits and specialized nursing care, potentially lowering overall treatment costs.
1Bortlik et al., J Crohns Colitis. 2013 Oct;7(9):736-43
Intract is a biopharmaceutical company developing disruptive oral antibody delivery solutions to significantly improve the efficacy and safety of emerging and established protein therapeutics, as well as improve patient experience and outcomes in inflammation and immunology indications. Its platform leveragesthe advantage of precision targeting large proteins and antibodies to the colon, while also protecting the biologics from enzymatic breakdown, allowing tissue/systemic uptake to create next-generation oral antibody medicines. For more information, please visitwww.intractpharma.com
Tharimmune is a clinical-stage biotechnology company developing a diverse portfolio of therapeutic candidates in immunology, inflammation and oncology. Its lead clinical asset, TH104, aims to suppress chronic pruritus associated with primary biliary cholangitis (PBC), a rare autoimmune liver disease with no known cure. The expanded pipeline includes TH023, an oral TNF-alpha inhibitor offering a new approach to treating autoimmune diseases. Tharimmune is also advancing early-stage multispecific biologics targeting unique epitopes against multiple solid tumors through its proprietary EpiClick™ Technology. The company has a license agreement with OmniAb, Inc. to access their antibody discovery technology for targeting specified disease markers. For more information, please visit: www.tharimmune.com.
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, contained in this press release, including statements regarding the timing and design of Tharimmune’s future Phase 2 trial, Tharimmune’s strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “depends,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “target,” “should,” “will,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that may cause such differences, include, but are not limited to, those discussed under Risk Factors set forth in our Annual Report on Form 10-K for the year ended December 31, 2023 and other periodic reports filed by the Company from time to time with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company’s views as of the date of this release. Subsequent events and developments may cause the Company’s views to change; however, the Company does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this release.
Dr Vipul Yadav
CEO
Tirth T. Patel
212-201-6614
LONDON and BRIDGEWATER, N.J. and / ACCESSWIRE / September 16, 2024
Tharimmune, Inc. (NASDAQ: THAR) (“Tharimmune” or the “Company”), a clinical-stage biotechnology company developing a portfolio of therapeutic candidates in inflammation and immunology, announced today that it has entered into a definitive agreement with Intract Pharma to exclusively license INT-023/TH023, an oral anti-Tumor Necrosis Factor-alpha (TNF-α) monoclonal antibody infliximab. This strategic partnership aims to expand Tharimmune’s therapeutic pipeline and reinforce its commitment to pioneering novel treatments for autoimmune diseases.
Under the terms of the agreement, Tharimmune licensed global development and commercialization rights (outside of South Korea) to Intract Pharma’s Soteria® and Phloral® delivery platform along with an existing supply agreement for infliximab to be used in the oral product development program. Intract received a single-digit million dollar upfront payment and is eligible to receive more than $200m on future development, regulatory and commercial milestones, as well as mid-single digit royalties based on net product sales. The agreement retains the right of first refusal to continue development and commercialization after a Phase 2 clinical trial. In addition, Tharimmune has the option to exercise the license to Intract’s platform for up to four additional targets.
Infliximab is a purified, recombinant DNA-derived chimeric IgG monoclonal antibody protein that contains both murine and human components that inhibit tumor TNF-α. Tumor Necrosis Factor- alpha is a signaling protein involved in acute phase reactions and systemic inflammation. Infliximab is sold by Janssen Biotech under the Remicade® brand for numerous indications including Crohn’s disease, ulcerative colitis, rheumatoid diseases and plaque psoriasis.
Traditionally administered through intravenous infusions, oral delivery of antibodies such as infliximab is challenging due to the complexity of navigating such large molecules through the gastrointestinal tract. This new partnership aims to overcome these challenges using Intract’s delivery platform, making it possible to administer infliximab in a pill form. An oral route of administration holds potential to improve patient compliance and quality of life, while also reducing the burden on the healthcare system associated with long-term intravenous therapy.
“The integration of Intract’s innovative technology, combined with the high-quality infliximab monoclonal antibody provided by their existing supply agreement, holds tremendous potential to accelerate and reinforce our commitment to immunology. “This new collaboration not only broadens our therapeutic focus, but also aligns with our mission to improve patient outcomes by developing more convenient and accessible treatment options,” said Randy Milby, CEO of Tharimmune. “An oral form of infliximab represents a significant advancement in the treatment of chronic inflammatory diseases, and the opportunity for Tharimmune to compete in a multibillion- dollar global market.”
This partnership enables the targeted delivery of antibody therapeutics directly to the colon or small intestine. By leveraging Intract’s platform, Tharimmune aims to enhance the effectiveness of TNF-α inhibitors such as infliximabthrough precision delivery that maximizes proteolytic stabilization and tissue permeation. This novel approach offers significant potential for directly addressing inflammatoryconditions within the gastrointestinal tract, including inflammatory bowel disease, as well as systemic inflammatory disorders where TNF-α plays a critical role in disease progression.
“Safer and orally available biological treatments for long term use in chronic inflammation and immune mediateddiseases represents a major area of medical need for millions of patients” said Vipul Yadav, CEO of Intract. “We are delighted to be partnering with Tharimmune and bringing on board their clinical development expertise in immunology to further advance together our oral anti- TNFα antibody into the clinic.”
About Intract Pharma
Intract is a biopharmaceutical company developing disruptive oral antibody delivery solutions to significantly improvethe efficacy and safety of emerging and established protein therapeutics, as well as improve patient experience and outcomes in inflammation and immunology indications. Its platform leverages the advantage of precision targeting oflarge proteins and antibodies to the colon, while also protecting the biologics from enzymatic breakdown, allowing tissue/systemic uptake to create next-generation oral antibody medicines. For more information, please visit www.intractpharma.com
About Tharimmune
Tharimmune, Inc. is a clinical-stage biotechnology company developing a portfolio of therapeutic candidates for inflammation and immunology. The Company’s lead clinical-stage asset, TH104, is known to suppress chronic, debilitating pruritus or “uncontrollable itching” in PBC, a rare and orphan liver disease with no known cure. The Company’s early-stage immunology pipeline includes novel multi-specific antibodies targeting unique epitopes with novel mechanisms of action against well-known, validated targets in multiple solid tumors, including PD-1, HER2 and HER3. Tharimmune has a license agreement with OmniAb, Inc. to access the company’s antibody discovery technology platform against these and other specified targets. For more information, please visit www.tharimmune.com.
Contacts:
Intract Pharma
Tharimmune, Inc.
LHA Investor Relations Tirth T. Patel [email protected]
212-201-6614
Intract Pharma London, UK (“Intract”) has today announced a publication on the collaboration with Merck & Co., Inc., Rahway, NJ, USA, on the pre-clinical proof-of-concept study evaluating colon targeted delivery of JAK inhibitor using the Phloral® technology in the journals of Pharmaceutics (https://www.mdpi.com/1999-4923/14/11/2385).
The study demonstrated the validation of gastric emptying and lower GI release of enteric Phloral coated capsules in a rat model, enhanced tissue PK and reduced systemic PK of tofacitinib when delivered to the lower GI compared to immediate-release formulations which led to superior downregulation of proinflammatory IL6 in a LPS induced rat colitis model.
The research conducted over 3 years between scientists from Intract and Merck & Co. successfully demonstrates the potential for oral targeted delivery of JAK inhibitor to achieve improved efficacy through enhanced colon tissue concentration, and better safety profile by minimizing systemic exposure since JAK inhibitors have been linked to serious cardiovascular and cancer events1.
“This research is another example of how targeted delivery of commercially available therapies can improve upon their safety and efficacy currently being achieved by conventional formulations,” said Vipul Yadav, CEO at Intract Pharma. “We look forward to building further on this research and validating the concept in large animals and eventually in patients suffering from moderate-to-severe ulcerative colitis where there is a critical need for safer JAK inhibitor medicines to allow improved patient access.”
About Intract Pharma
Intract Pharma is biopharmaceutical company bringing disruptive oral small molecule and biologics delivery solutions to significantly improve the efficacy and safety of emerging and established biotherapeutics and improve patient experience and outcome. The platform leverages the advantage of delivering large proteins/antibodies to the colon, while also protecting the biologics from enzymatic breakdown allowing tissue/systemic uptake to create next generation oral biologic medicines. For more information visit us at www.intractpharma.com.
Intract Media Contact
Dr Vipul Yadav
CEO
AB-BIOBETTER, led by Bio-Sourcing, to pioneer development of new oral formulation of adalimumab for inflammatory bowel diseases; offering patients less invasive, more cost-effective and potentially more potent treatment options
Liège (Belgium), Montpellier (France) and London (UK), May 28, 2024 – Bio-Sourcing, with partners Ciloa and Intract Pharma, today announce the launch of a €3.4M ($3.7M) EU-funded program, including €1.9M in subsidies from EUREKA Eurostars. The three-year AB-BIOBETTER project aims to develop an oral antibody treatment for Inflammatory Bowel Diseases (IBD), such as Crohn’s disease and ulcerative colitis, as well as for other systemic immunology indications. Bio-Sourcing, a Belgian biotechnology company developing a new platform for the sustainable production of a new generation of biotherapeutics, is the lead coordinator of the project. Its partners are Ciloa, a French preclinical stage biotechnology company and pioneer in the field of exosomes (a subpopulation of extracellular vesicles (EV)) bioengineering, and Intract Pharma, a UK developer of disruptive oral antibody delivery solutions.
AB-BIOBETTER was selected during the Eurostars call for projects. It is co-financed by the national budgets of each partner’s state, via the Public Service of Wallonia for the Economy, Employment and Research, Bpifrance and Innovate UK, as well as by the European Union. It aims to develop a daily pill-based version of adalimumab, an anti-TNF-α monoclonal antibody, for the treatment of patients with inflammatory diseases, including IBD. The goal is to offer a practical, less invasive and less expensive alternative to the subcutaneous injections currently used, with equal or even improved efficacy.
“We are delighted to work with Ciloa and Intract Pharma on this pioneering project in the oral delivery of monoclonal antibodies, as recognized by the European Union. Combined with the intrinsic benefits of our technological platform, it will make these essential medicines even more accessible, especially for low- and middle- income countries which are currently deprived of them,” said Bertrand Merot, CEO and founder of Bio-Sourcing.
AB-BIOBETTER aims to meet this challenge by combining cutting-edge technologies from Bio-Sourcing, Ciloa and Intract Pharma. Bio-Sourcing’s BioMilk platform enables the production of an adalimumab biobetter – a new and improved version of existing biologics – in industrial quantities. It should cost ten times less than conventional monoclonal antibodies. The generation of antibodies formulated in milk-derived extracellular vesicles could potentially be better suited to oral delivery. Thus, Bio-Sourcing’s platform offers competitive and disruptive advantages for antibody therapeutics.
Ciloa brings to the project its expertise in extracellular vesicle bioengineering, which will enable accurate and effective oral administration of the drug. Intract Pharma will contribute with its precision gut delivery and antibody stabilization technologies, allowing for the oral delivery of biotherapeutics.
“I often say that what can be imagined with proteins and extracellular vehicles can be realized and this is one of those opportunities,” said Robert Mamoun, CEO and co-founder of Ciloa. “We look forward to working with our partners to bring this innovative treatment to market and improve the lives of patients living with chronic inflammatory diseases. The success of this endeavor will represent a breakthrough in the oral formulation of biotherapeutics.”
‘’We are excited to be part of this innovative project to evaluate the potential of milk-derived extracellular vesicles for the oral delivery of antibody therapeutics, which remains the holy grail in biotherapeutics delivery. The deployment of each platform and our combined expertise will allow us to double down on the goal of making antibody therapeutics available in pill form. This would transform the current patient treatment paradigm in chronic inflammatory and immune-mediated diseases,” said Vipul Yadav, CEO of Intract Pharma.
The three-year long project aims to preclinically validate the product and the platform, and to bring the product up to the IND-enabling studies stage.
Eurostars is a part of the Horizon Europe program that supports innovative SMEs and project partners (universities, research organizations and others) by funding international collaborative R&D and innovation projects. Eurostars is run by EUREKA, an intergovernmental network involving 37 countries.
About Ciloa
Ciloa is a French preclinical stage biotechnology company and pioneer in the field of extracellular vesicle bioengineering (EV). The company aims to create new generations of EV-based biotherapies and vaccines. Its proprietary technology allows the in vivo editing by cells of EV, with all types of proteins targeted to the membrane and/or in the lumen.
Ciloa has world-class expertise and proprietary processes enabling high-yield production of high-purity and stable EV. Its quality control processes guarantee characterization of EV according to the ‘state of the art’. Thanks to its process and its clean room facility, Ciloa produces GMP-like EV for regulatory preclinical studies.
About Intract Pharma
Intract Pharma is developing disruptive oral antibody delivery solutions to significantly improve the efficacy and safety of emerging and established protein therapeutics, as well as improve patient experience and outcomes in inflammation and immunology indications. Its platform leverages the advantage of precision targeting of large proteins and antibodies to the colon, while also protecting the biologics from enzymatic breakdown, allowing tissue/systemic uptake to create next-generation oral antibody medicines.
About Bio-Sourcing
Bio-Sourcing is a biotechnology company that has developed a unique, sustainable and profitable platform to produce a new generation of biotherapeutics; in particular, monoclonal antibodies. Its technology platform is based on the production of biotherapeutics in goat milk using genome editing and nuclear transfer technologies.




Press and analyst contacts
Andrew Lloyd & Associates
Juliette Schmitt / Saffiyah Khalique
Tel: +44 1273 952 481
Intract Pharma Limited London, UK (“Intract”), and Elasmogen Limited, Aberdeen, Scotland (“Elasmogen”) have announced a global license agreement that gives Elasmogen exclusive access to Soteria® and Phloral® technologies for oral colonic delivery of therapeutic proteins, deriving from Elasmogen’s soloMER™ platform, for an undisclosed target for treatment of Crohn’s disease and ulcerative colitis.
The license agreement builds upon the research collaboration the two companies entered in to in May 2020 that involved pre-clinical evaluation of Soteria® and Phloral® technologies utilising Elasmogen’s multi-valent, novel inflammatory cytokine binding soloMER™ protein therapeutics. Acceleration of this research and development effort is facilitated by a recent £8M funding round announced by Elasmogen and a £1.5M Innovate Future Leaders grant. This grant funding in particular is specifically focused on Elasmogen’s auto-immune programs.
Under the terms of the agreement, Intract will receive an undisclosed upfront payment followed by development milestone payments and royalties on product sales. Elasmogen will have the option to expand the license agreement for up to four additional targets.
‘’We are excited to build on our ongoing partnership with Elasmogen to create new transformative delivery options for innovative protein medicines to meet the unmet needs of millions of patients suffering from chronic inflammatory bowel disease,’’ said Vipul Yadav, CEO at Intract Pharma. ‘’We believe oral targeted protein medicines are the future of IBD therapies and our collaboration with Elasmogen keeps building on our mission to bring innovative oral biologic therapies to patients.’’
“Securing this agreement with Intract enables us to deliver our incredibly potent anti-inflammatory soloMERs directly where they are needed in the GI tract, minimizing off-site adverse effects and maximizing clinical outcomes” said Caroline Barelle, CEO of Elasmogen. “We are really looking forward to working with the Intract team to expand our growing, differentiated pipeline of therapeutic biologics”.
About Intract Pharma
Intract Pharma is biopharmaceutical company bringing disruptive oral biologics delivery solutions to significantly improve the efficacy and safety of emerging and established biotherapeutics and improve patient experience and outcome. The platform leverages the advantage of delivering large proteins/antibodies to the colon, while also protecting the biologics from enzymatic breakdown allowing tissue/systemic uptake to create next generation oral biologic medicines. For more information visit us at www.intractpharma.com.
About Elasmogen
Elasmogen is a privately held biopharmaceutical company located in the thriving biologics cluster in Aberdeen, Scotland. The company is rapidly progressing a pipeline of next-generation soloMER products for the treatment solid-tumour cancers, systemic inflammatory diseases and inflammatory conditions of the gut. The company’s proprietary technology is protected by a robust and layered IP portfolio covering the generation, humanization, half-life extension, products and product formats.
Intract Media Contact
Dr Vipul Yadav
CEO
Elasmogen Media Contact
Dr Caroline Barelle
CEO
April 1st, 2022
London, April 01, 2022 – Intract Pharma, an innovative oral protein delivery company, today announced the appointment of Dr Vipul Yadav as Chief Executive Officer of the company, effective April 1st, 2022. Dr Yadav, who has been serving as the Director of Research for over 5 years, also joins Intract Board of Directors. Dr Bill Lindsay who co-founded and led Intract since incorporation in 2015 will remain with the company as Director of Business Development.
On the appointment, Bill Lindsay said “It is with great satisfaction that I hand over management of Intract to Dr Yadav, who has been a major driving force in development of the company to date, in particular in pioneering our biotherapeutics development platform, Soteria”.
Dr Yadav said ‘’I am excited to transition into this role at such a critical junction of the company’s journey towards progressing its oral protein technology platform into clinical development with multiple pharma partners. I would like to thank the current management and board for entrusting me with this role and I look forward to working alongside the Intract board, management, and R&D staff to lead the company to the next phase of growth and realise the potential of our innovative technologies’’.
Dr Vipul Yadav joined Intract following completion of his PhD and since then has served as the Director of Research. During his tenure the company advanced into the oral biologic delivery space for treatment of gastrointestinal and systemic immunoinflammatory diseases and established multiple pharma partnerships to advance the technology deeper into preclinical with early and late-stage biologic candidates.
Dr Yadav received his PhD from University College London, School of Pharmacy working in the lab of Intract founder Prof Abdul Basit where he researched gastrointestinal stability and tissue uptake mechanisms involved in oral delivery of peptides and monoclonal antibodies.
London, UK. September 27th 2021.
GUANGZHOU, China, September XX, 2021 (BUSINESSWIRE) – Bio-Thera Solutions, Ltd. (688177.SH, “Bio-Thera”) and Intract Pharma (“Intract”) announced a global collaboration and licensing agreement that gives Bio-Thera access to Intract’s Soteria® and Phloral® drug delivery technologies to develop novel oral monoclonal antibody (mAb) treatments for chronic gastrointestinal (GI) inflammatory diseases. Bio-Thera is a global pharmaceutical company developing innovative therapeutics and biosimilars for oncology, autoimmune diseases, cardiovascular diseases, and other serious unmet medical needs.
Under the terms of the agreement, Intract has granted Bio-Thera a worldwide license to Intract’s oral biologics drug delivery platform for a single undisclosed mAb product. Intract will receive an undisclosed upfront payment, with potential development and commercial milestone payments along with royalties on product sales. Intract will lead preclinical research of the product and Bio-Thera will have the option to expand development of the product for multiple GI indications and will be responsible for manufacturing and commercialization of any approved products.
Intract’s oral biologics delivery platform includes Soteria® a technology which protects proteins such as mAbs from degradation in the intestinal lumen. The platform also includes Phloral®, a clinically-validated technology that utilizes both pH and the enzymes produced by colonic bacteria as a trigger mechanism to allow accurate release of payloads in the colon, a suitable site in the GI tract for targeting of biologics for local and systemic delivery.
“Developing oral biologics is the next step in advancing compliance and access to innovative monoclonal antibodies for chronic diseases,” said Dr. Shengfeng Li, CEO of Bio-Thera Solutions. “We believe that combining Bio-Thera’s antibody expertise with Intract’s oral delivery platform will lead to a great advance in the treatment of gastrointestinal disease.”
“Intract Pharma is delighted to be partnering with Bio-Thera to address the significant challenge of delivering powerful biologics orally” said Dr Bill Lindsay, CEO of Intract. “We believe that our technologies will allow development of a more effective therapeutic for the treatment of IBD, while also increasing drug safety and reducing cost.”
About Bio-Thera Solutions, Ltd.
Bio-Thera Solutions, Ltd., a leading commercial-stage biopharmaceutical company in Guangzhou, China, is dedicated to researching and developing novel therapeutics for the treatment of cancer, autoimmune, cardiovascular diseases, and other serious unmet medical needs, as well as biosimilars for existing, branded biologics to treat a range of cancer and autoimmune diseases. As a leader in the next generation antibody discovery and engineering, the company has advanced six candidates into late-stage clinical trials and one of which, QLETLI® (格乐立®), a biosimilar to adalimumab, is available to patients in China. In addition, the company has multiple candidates in early stage clinical or entering clinical studies, including differentiated and innovative anti-OX40, anti-TIGIT, and anti-PD-L1/CD47 bispecific antibodies. For more information, please visit www.bio-thera.com/en/ or follow us on Twitter (@bio_thera_sol) and WeChat (Bio-Thera).
About Intract Pharma
Intract Pharma is a biotechnology company bringing disruptive oral biologics delivery solutions to significantly improve the efficacy and safety of emerging and established biotherapeutics and improve patient experience and outcome. The platform leverages the advantage of delivering large proteins/antibodies to the colon, while also protecting the molecules from enzymatic breakdown, allowing tissue/systemic uptake to create next generation oral biologic medicines. For more information visit us at www.intractpharma.com.
Bio-Thera Solutions Cautionary Note Regarding Forward-Looking Statements
This news release contains certain forward-looking statements relating to Bio-Thera Solutions’ collaboration with Intract or product pipelines in general. Readers are strongly cautioned that reliance on any forward-looking statements involves known and unknown risks and uncertainties. The forward-looking statements include, among others, those containing “could,” “may,” “should,” “will,” “would,” “anticipate,” “believe,” “plan,” “promising,” “potentially,” or similar expressions. They reflect the company’s current views with respect to future events that are based on what the company believes are reasonable assumptions in view of information currently available to Bio-Thera Solutions and are not a guarantee of future performance or developments. Actual results and events may differ materially from information contained in the forward-looking statements as a result of a number of factors, including, but not limited to, risks and uncertainties inherent in pharmaceutical research and development, such as the uncertainties of pre-clinical and clinical studies, for example, the development processes could be lengthy and in vitro or early, small scale clinical trial results may not translate into desired results in vivo or in large scale clinical studies. Other risks and uncertainties include challenges in obtaining regulatory approvals, manufacturing, marketing, competition, intellectual property, product efficacy or safety, changes in global healthcare situation, changes in the company’s financial conditions, and changes to applicable laws and regulations, etc. Forward-looking statements contained herein are made only as of the date of their initial publication. Unless required by laws or regulations, Bio-Thera Solutions undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, changes in the company’s views or otherwise.
Bio-Thera Solutions Media Contact
Bert E. Thomas IV
Senior Vice President, Business Development
+1 410 627 1734 (mobile) [email protected]
Intract Media Contact
Bill Lindsay
CEO
London, UK. April 1st 2021.
Intract Pharma Limited today announced the initiation of a collaborative research programme with Ferring Pharmaceuticals, to investigate oral delivery of a monoclonal antibody (mAb) targeting an undisclosed interleukin for treatment of inflammatory bowel disease (IBD). The research will focus on evaluation of Intract’s Phloral® and Soteria® technologies for precision oral delivery of the therapeutic mAb directly to GI tissue to improve efficacy and minimize systemic exposure.
Commenting on the collaboration, Bill Lindsay, Intract’s CEO, said “Intract’s technologies are unique in terms of precision targeting of biologic drugs, especially mAbs, via the oral route allowing the possibility for localized treatment of GI diseases without the risk of serious adverse effects resulting from high dose injections. We are excited to enter this collaboration with Ferring which has a long heritage in gastroenterology products and a commitment to continuous innovation for new therapies for patients suffering from chronic GI diseases”.
About Intract Pharma
Intract Pharma is a company which specializes in oral drug delivery, developing state-of-the-art formulation technologies and unique gastrointestinal models to develop advanced new therapeutics. Since its inception, Intract has pursued innovation in oral drug delivery as a means to improve on current therapies, making them safer and more effective for patients. The drug delivery technologies Phloral® for precise colonic drug delivery, and Soteria® for delivery of biotherapeutics to gastrointestinal tissue, are available for license exclusively from Intract Pharma. For more information visit www.intractpharma.com
Copyright © 2018 Intract Pharma
A site by: Web Design London