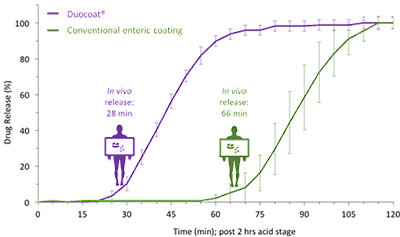
In vitro dissolution testing is a critical tool to control the quality of pharmaceutical products and guide formulation development. However, traditional approaches are often crude, inaccurate measures of actual intestinal release behavior in humans.
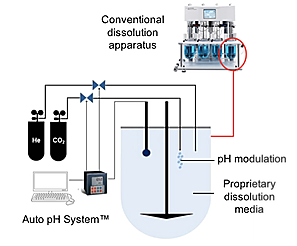
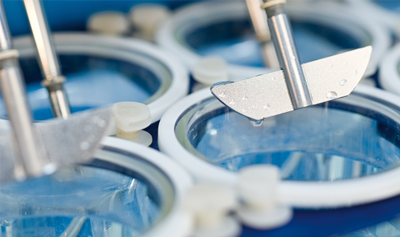
The Dynamic Dissolution Model builds upon conventional apparatus but incorporates a unique bicarbonate-based media controlled by a patented Auto pH System™. These innovative features characterize the dynamic environment of the GI tract and modulate parameters crucial to dissolution and drug release. This provides greater ability to accurately discriminate between formulations.